Implanting an ICD
Before the procedure
Before the procedure, you will need to take some blood tests, e.g. to check for infections and cell counts. If you have any bleeding disorder or if you are under medication with blood-thinning drugs, please let your doctor know; you may be asked to pause some of these medications before the procedure. If you have any allergies or in case you are/ may be pregnant, you should inform your doctor.
The day before the procedure, you may be asked to wash your whole body with a special soap. You will also be requested to fast for some time, usually overnight, as sedatives or general anesthesia might be required during the procedure which can only be given safely when your stomach is empty. This includes any food or drink (inclusive water, chewing gums or smoking). If you should take some medications in the morning before the procedure, you should take them with a small sip of water.
Other specific preparation can sometimes be necessary, depending on your general medical condition.
During the procedure
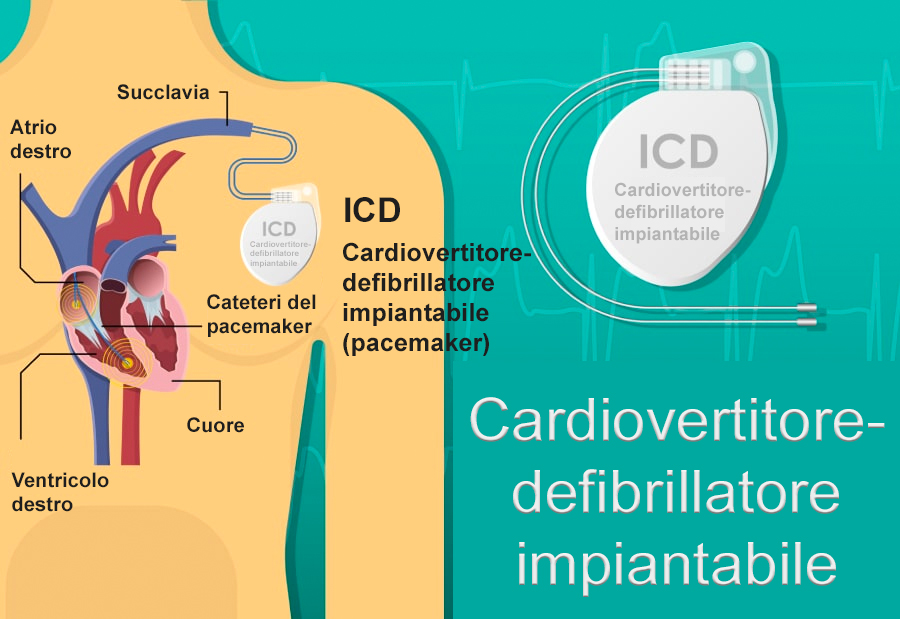
For transvenous ICDs, the implantation procedure is very similar to that of a pacemaker.
The procedure can be performed under local anesthesia with general sedation or, sometimes, under general anesthesia. The operation consists of incising the skin of the chest over 5 to 10 centimeters in the chest muscle region below the collarbone, in the vast majority of cases on the left but sometimes, exceptionally, on the right. In special situations (e.g. being an athlete or hunter), special implantation places or techniques can be used. Talk to your doctor, if you think this could be the case with you. The incision is used to approach the veins that will guide the leads towards the heart and install the box under the skin or under the muscle. The procedure takes about an hour and can take place in an operating theater or in an electrophysiology room (EP lab). The leads are guided towards the heart using a fluoroscopy (X-ray) device. The ICD box is connected to the leads and the skin is then closed by sutures or surgical glue. It is not uncommon for a compression dressing to be put in place for the postoperative period. Then, it is only a simple dressing on the scar that will be kept for several days until complete healing.
For the subcutaneous ICD, the incision is larger and is made at the side of the chest, below your arm for placement of the box and the lead is guided under the skin of the chest horizontally relative to the box and then vertically. The same precautions are taken on the scar.
After the procedure
After the procedure, you will be taken at your hospital room or at the recovery room for observation during some hours. Your heart rhythm and blood pressure will be monitored by a nurse. If you feel chest pain, dizziness or other discomfort, you should notify the nurse immediately. After a few hours, you may get of your bed carefully, with the assistance of the nurse. Once the observation time is completed and you recover, you may eat and drink.
You may be discharged from the hospital at the same day or the day after. Your device should be checked before discharge to make sure it works properly. If you are discharged the same day, you should have someone to accompany you home, due to the sedative drugs taken during the procedure. Before discharge, ask for instructions on how to manage your device, as well as advice on follow-up appointments, exercise, medication, care and resumption of normal activities.
The incision site can be painful the first days after the procedure and painkillers may be needed. You will also experience itching skin around the wound for some time after surgery, which is quite normal. The incision should be kept dry and clean (your nurse should give you more instructions before discharge). Before discharge from the hospital, you will be given instructions how to treat the incision to reduce the chance of an infection and allow proper healing.
Your doctor (or nurse) will check your device a couple of weeks after surgery to make sure the device works properly and there are no signs of complications. Nevertheless, you should contact your doctor in case you experience any of the following after device implantation.
- Symptoms or complaints you also felt before device implantation.
- When you encounter blackouts/fainting spells.
- When you experienced a shock of the ICD.
- In case of increased shortness of breath, chest pain or dizziness.
- Direct contact is necessary when there is a suspicion of an infection, for example in case of fever (>38,5gr C), chills or sweats, redness or swelling of the device pocket or drainage or progressive pain from the incision site.
- In case of bleeding from the incision site or when the incision edges begin to pull apart.
- In case of an alert, some devices than can either beep or vibrate. Ask your physician or nurse if your device has this capability; if it does, they can give you a demonstration of the sound/vibration, so you are better prepared.
If not stated otherwise by your doctor, you should be able to return to your job and daily activities within a couple of weeks. You do not need to restrict arm movements and/or use an arm sling after device implantation, as this has not been shown to reduce risk of lead dislodgment and may increase shoulder pain. Before resuming your exercises/sports, it is best to consult your doctor for advice. If your stitches need to be removed, your doctor will inform you when and where to do it. Regarding driving, you should ask your doctor for instructions.
Routine follow-ups

Once you have a cardiac device, you will need to have regularly follow-ups by a doctor, or a nurse specialized on device programming.
The first follow-up is usually scheduled 2-12 weeks after the implantation of the device. The doctor will inspect the incision site to check if it is closed properly without any signs of infection. Then the doctor will perform a technical control, using a specific computer (programmer) which will connect to your device.
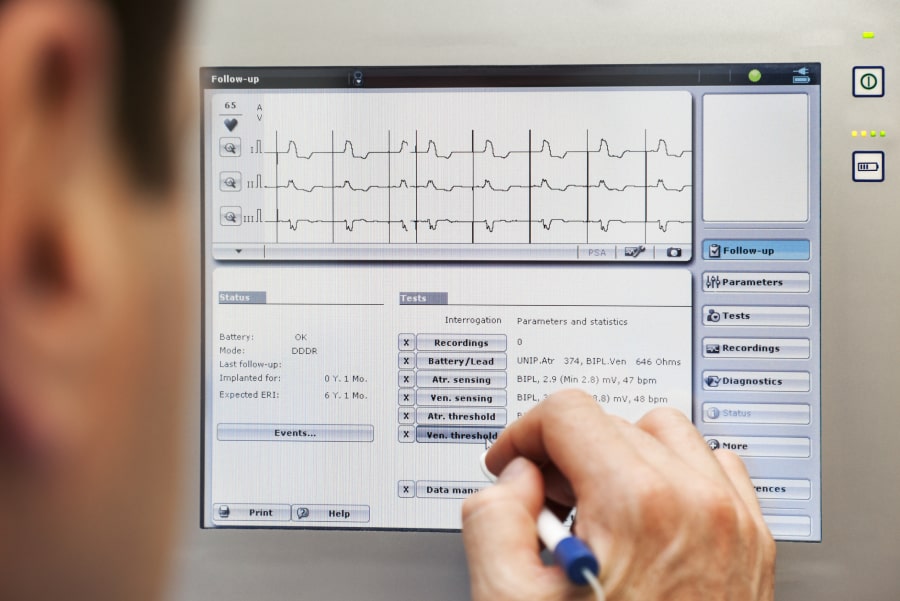
During the technical check, the doctor or technician will review the data recorded during the follow-up period. That includes technical data regarding the electrical properties of the leads, as well as clinical information about you (your mean heart rate and heart rate distribution, how much time has each lead of your pacemaker been working). The device can also detect tachycardias and in many times the arrhythmia can be recorded and stored.
The doctor or technician can make several tests to confirm the appropriate function of the leads. You may briefly feel your heart rhythm go faster (palpitation) or slower than normal (can feel like dizziness), which can cause some discomfort for a few seconds. This is totally normal and will disappear as soon as the test is finished. The doctor or technician may change some settings of your device during the follow-up to make the device more effective.
After the first follow-up, you will be scheduled to regular follow-ups once per year or more often if needed.
Remote monitoring of cardiac devices has been developed for technical check-ups using transtelephonic data transmission. In addition, automatic or patients triggered alerts to the cardiologist or technician can respond, if necessary, with various interventions. Remote monitoring is also capable to send information from the device on the programmable features, device function, arrhythmias, cardiovascular hemodynamic function and registration of stored arrythmias.
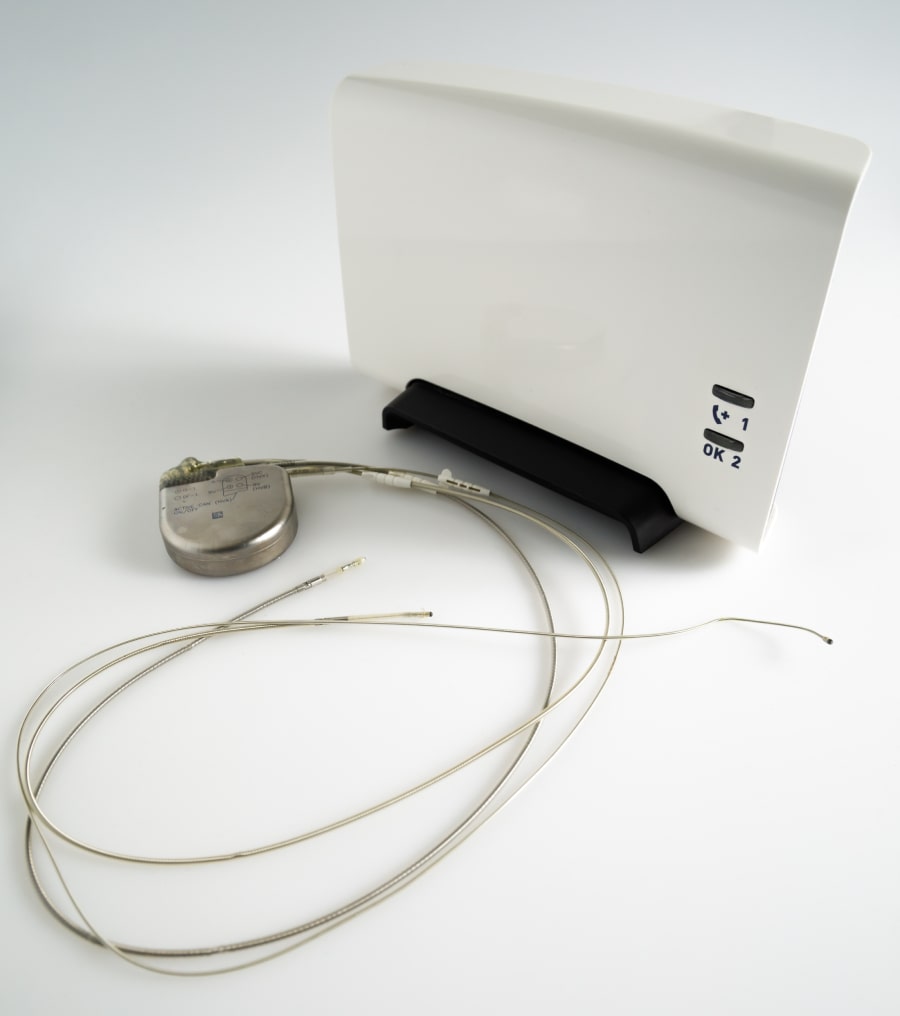
It should be noted that the transmitter has to be in the proximity of you to be able to communicate with the device. When the transmitter has received the data from the cardiac device it sends its data encrypted to the central server of the manufacturer of the device so it becomes accessible for the cardiologist or technician to evaluate. Remote monitoring cannot reprogram the device avoiding any potential threat for the patient. Check with your hospital or cardiologist to see how you will be informed after a remote monitoring is performed.
Your device may sometimes have a sound or vibration alarm. In such a case you should get in contact with your physician / pacing department asking for further instructions. During your routine follow up, you may also ask your technician/physician if your device as such a feature and what to if it happens
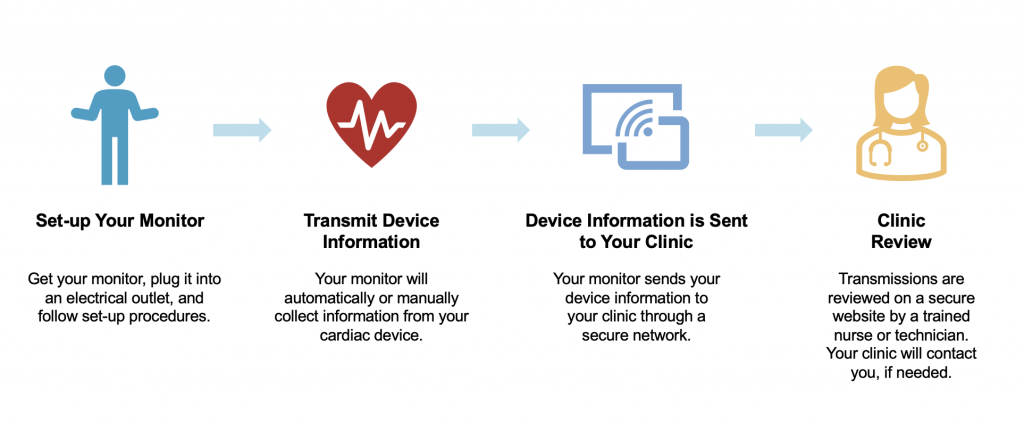 Currently, remote monitoring is expanding rapidly across Europe but the methodology and practicality varies considerably between countries and hospitals.
Currently, remote monitoring is expanding rapidly across Europe but the methodology and practicality varies considerably between countries and hospitals.
What does my device ID tell me/how to read my device ID?
The device identification card contains besides your personal information and contact details of your hospital, information of the cardiac device. Therefore, it is important for you to always carry this ID card.
The details of the cardiac device contain information on the type and the manufacturer of the device, either pacemaker or ICD including model and serial number, the type and manufacturer of the leads and date of device implantation.
Generator change
When the battery of the device reaches the end of its life (usually an average of 7-10 years, but may vary, sometimes more, sometimes less), the generator needs to be changed. If the leads are intact and work perfectly, there is no need to replace them. The battery of the device will not end suddenly and unexpectedly. From the time it reaches what is called elective replacement indicators, it usually has at least 3 months of extra battery lifetime. This means that there is plenty of time to plan for a change.
The preparation for the replacement of the generator is similar with the preparation for a device implantation (see above). The procedure, however, is much simpler with fewer complications if no leads need to be added or replaced. Your physician will determine whether the same incision can be used or a new incision needs to be created. If you have concerns about having multiple scars, please do mention this to your physician.The old generator will be disconnected from the leads and will be replaced by the new one in the same place. The new generator will then be connected to the electrodes. The device will be programmed and then the incision will be closed with sutures.
The observation time after the procedure is usually short and you will be probably discharged the same day.
What are the risks and Complications
A pacemaker or ICD implantation is a routine procedure in most hospitals; thus, the complications are infrequent. The most important complications are summarized below:
- Pneumothorax/ hemothorax: This is a condition where the lungs can accidentally be damaged during the procedure, resulting in air (pneumothorax) or blood (hemothorax) collecting in the sac around the lungs. It is a very rare complication. If it happens, a drainage will be placed to resolve the air or blood.
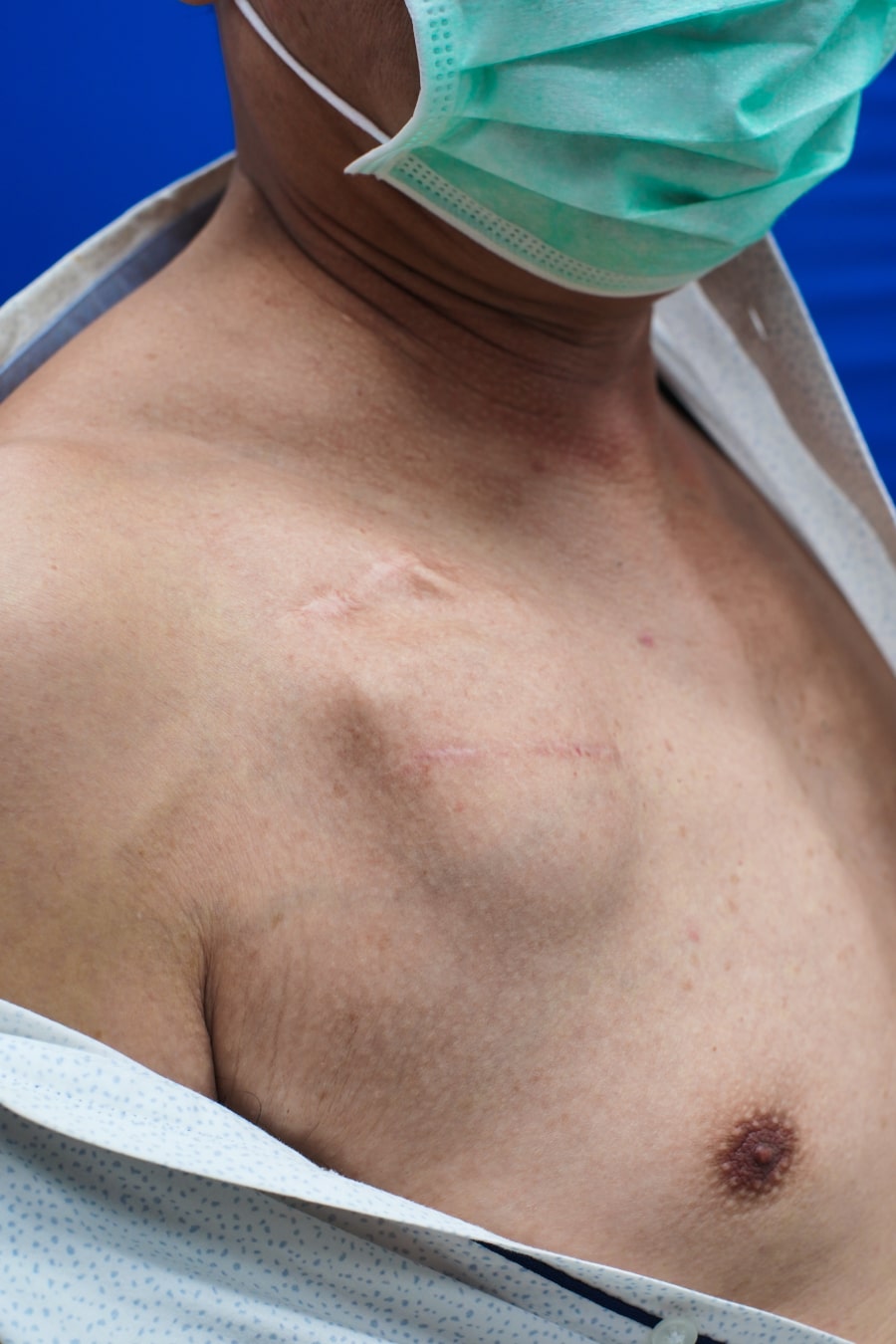
- Swelling, bruising or bleeding at the incision site on the chest. Such a complication is rarely severe, and usually occurs the first days after the procedure. It is more common among patients who are under medication with blood thinners.
- Infection at the incision site: Usually occurs the first weeks after the implantation but can also occur later. This is a severe complication that needs immediate treatment.
- Dislocation (movement) of the electrodes: This can occur either during or just after the procedure and be repaired immediately or long time after the implantation.
- Infection on the battery or leads: Fortunately, rare but a severe complication. Can appear long time after the procedure and need special treatment.
- Even though uncommon, damage to the nerves or blood vessels close to the pacemaker implantation site can occur, leading to local discomfort.
